Pain in Women - an unmet need throughout the world
Pain in Women
Before puberty, boys and girls have similar low rates of persistent pain conditions. However, by the age of 16-18 years, girls report 3-4 times the rate of pain in males, with the most common presentation being a combination of abdominal pain and headache in adolescent girls. Throughout life, chronic pain conditions are more common in females than males.
Pelvic pain is more than period pain (dysmenorrhea)
One in 5 young women suffer severe period pain with an unknown proportion proceeding to develop chronic pelvic pain over time. For those who do transition from period pain to chronic pelvic pain, this may ocurr over a relatively short time or only after many years. The timeline for progression from severe period pain to chronic pelvic pain was investigated by researchers within our team, with 16% of women with pelvic pain reporting transition to pelvic pain within 12 months of developing severe period pain and over 50% reporting transition within 12 years.
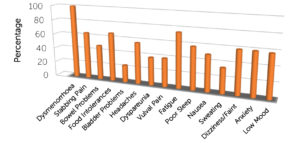
In 2018, Associate Professor Susan Evans published the symptom profile of 168 women with pelvic pain symptoms that included severe period pain. The presence or absence of 14 additional symptoms was recorded for each woman, and the findings are displayed in the figure below. There was an average of 8 symptoms present in each woman. The full article is available at the following link: jpr-179409-the-co-morbidities-of-dysmenorrhea-a-clinical-survey-compar-111418
Alyra Biotech's goal is to develop products that prevent the transition from severe period pain to chronic pelvic pain.
Pain conditions of the bladder or bowel
Pain conditions of the bladder and bowel are common. Irritable Bowel Syndrome affects between 7% and 21% of Americans with 2 in 3 of those affected being female, while 0.3% suffer the more severe Inflammatory Bowel Disorders. Bladder Pain Syndrome, also called Interstitial Cystitis affects between 3 and 8 million American women with 5 in 6 of those affected being female. These disorders are associated with increased immune activation and central sensitization. Although this cannot be tested in humans, it is anticipated that they also include activation of the glial cells within the dorsal horn of the spinal cord.
As with other pain conditions, current treatments are available. However, adverse effects and the need to take these medications daily make them unappealing to young women.
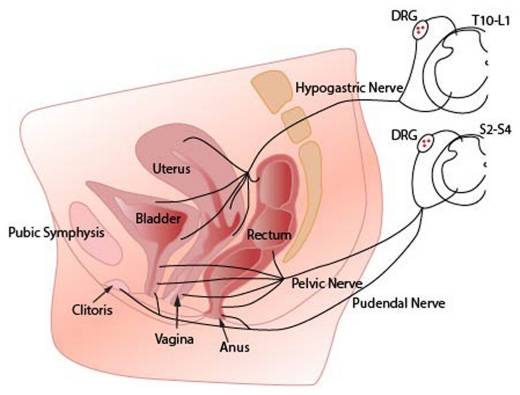
Following preclinical research demonstrating that activation of the immune system in the uterus results in excessive activation of spinal glial cells within the dorsal horn of the spinal cord (a condition seen in chronic pain), Alyra Biotech began development of intrauterine devices that will turn down this activation and thus reduced chronic pain. It is anticipated that these devices will also improve symptoms of an irritable bowel or painful bladder as the nerve supply to all three organs converges on the same segments of the spinal cord: Jobling P. Female Reproductive Tract Pain- targets challenges and outcomes
Our products use intrauterine administration of immune inhibitors to treat bladder and bowel symptoms by reducing spinal glial activation within the dorsal horn.
Medical conditions of the central nervous system that are more common in women
Women are over-represented in populations with conditions of the central nervous system associated with inflammation and activation of the innate immune system. These conditions may present in 3 ways:
- Certain medical conditions affecting the central nervous system are present in both men and women, but are known to be more frequent in females. For example, migraine headache occurs three times as commonly in women than men, affecting approximately 18% of women compared with 6% of men in the United States.
- Certain medical conditions occur only in the luteal phase of the menstrual cycle. For example, Premenstrual Syndrome (PMS) and Premenstrual dysphoric disorder (PMDD) describe a variety of affective, behavioural and somatic symptoms that occur in the luteal phase of the menstrual cycle during a woman’s reproductive years.
- Certain medical conditions may be present throughout the menstrual cycle, but be exacerbated in the days prior to a menstrual period. For example, established psychiatric diagnoses including persistent depressive disorder, major depressive disorder, bipolar disorder and anxiety disorders may be exacerbated premenstrually in a proportion of women.
Where there is an inflammatory component to the medical condition, with activation of circulating immune cells and associated cytokine release, a reduction in immune activation holds promise for effective management.
Our Lead Device
Although women using a levonorgestrel-releasing intrauterine device have reduced menstrual blood flow, a proportion of women using these devices report increased pain following insertion of their intrauterine device. Overall, between 4 and 14% of women request premature removal of the device, mostly due to pain. Even where the device is well tolerated, and menstrual pain is reduced, additional pain symptoms and pain-associated symptoms may persist. This is because these symptoms are associated with excess glial activation in the glial cells of the spinal cord. Our novel, non-opioid, products add an immune inhibitor to an intrauterine device to reduce this effect.
Patient Resources
The Pelvic Pain Foundation of Australia http://www.pelvicpain.org.au
References:
- Bush D, Evans S, Vancaillie T. “The 6 Billion Woman and the $600Million Girl: The Pelvic Pain Report,” 2011. http://www.fpm.anzca.edu.au/Pelvic_Pain_Report_RFS.pdf
- K N Dodds, E A H Beckett, S F Evans, P M Grace, L R Watkins, and M R Hutchinson. “Glial Contributions to Visceral Pain: Implications for Disease Etiology and the Female Predominance of Persistent Pain.” Translational Psychiatry 6, no. 9 (September 13, 2016): e888. https://doi.org/10.1038/tp.2016.168
- Evans, Jemma, and Lois A. Salamonsen. “Inflammation, Leukocytes and Menstruation.” Reviews in Endocrine & Metabolic Disorders 13, no. 4 (December 2012): 277–88. https://doi.org/10.1007/s11154-012-9223-7.
-
Evans Sf, Brooks TA, Esterman Aj, Hull Ml, and Rolan Pe. “The Comorbidities of Dysmenorrhea: A Clinical Survey Comparing Symptom Profile in Women with and without Endometriosis.” Journal of Pain Research, 2018, 3181–3194. https://doaj.org/article/d9e23b1430ff4d9386b56d3d2a2f6296.
-
Jobling, Phillip, Kate O’Hara, and Susan Hua. “Female Reproductive Tract Pain: Targets, Challenges, and Outcomes.” Frontiers in Pharmacology 5 (2014). https://doi.org/10.3389/fphar.2014.00017.
-
Hardi, Gemma, Susan Evans, and Meredith Craigie. “A Possible Link between Dysmenorrhoea and the Development of Chronic Pelvic Pain.” The Australian & New Zealand Journal of Obstetrics & Gynaecology 54, no. 6 (December 2014): 593–96. https://doi.org/10.1111/ajo.12274.
- Khan, K. N., M. Kitajima, K. Hiraki, A. Fujishita, T. Ishimaru, and H. Masuzaki. “Escherichia Coli in Menstrual Blood: An Association with Bacterial Endotoxin and Toll-like Receptor 4 (TLR4)-Mediated Growth of Endometriosis.” Journal of Reproductive Immunology 71, no. 2 (2006): 152–153. https://doi.org/10.1016/j.jri.2006.08.030.
- Khan, Khaleque Newaz, Akira Fujishita, Michio Kitajima, Koichi Hiraki, Masahiro Nakashima, and Hideaki Masuzaki. “Intra-Uterine Microbial Colonization and Occurrence of Endometritis in Women with Endometriosis †.” Human Reproduction 29, no. 11 (2014): 2446–2456. https://doi.org/10.1093/humrep/deu222.
- Kwok, Yuen H., Mark R. Hutchinson, Melanie G. Gentgall, and Paul E. Rolan. “Increased Responsiveness of Peripheral Blood Mononuclear Cells to In Vitro TLR 2, 4 and 7 Ligand Stimulation in Chronic Pain Patients (Increased TLR Responsiveness in Pain Patients)” 7, no. 8 (2012): e44232. https://doi.org/10.1371/journal.pone.0044232.
- Yuen H Kwok, Jonathan Tuke, Lauren L Nicotra, Peter M Grace, Paul E Rolan, and Mark R Hutchinson. “TLR 2 and 4 Responsiveness from Isolated Peripheral Blood Mononuclear Cells from Rats and Humans as Potential Chronic Pain Biomarkers.” PLoS ONE 8, no. 10: e77799. Accessed December 28, 2018. https://doi.org/10.1371/journal.pone.0077799.
- Lacagnina, Michael J., Linda R. Watkins, and Peter M. Grace. “Toll-like Receptors and Their Role in Persistent Pain.” Pharmacology & Therapeutics 184 (April 2018): 145–58. https://doi.org/10.1016/j.pharmthera.2017.10.006
- Erin D. Milligan, and Linda R. Watkins. “Pathological and Protective Roles of Glia in Chronic Pain.” Nature Reviews Neuroscience 10, no. 1 (2009): 23–36. https://doi.org/10.1038/nrn2533.
- Nicotra, Lauren, Lisa C. Loram, Linda R. Watkins, and Mark R. Hutchinson. “Toll-like Receptors in Chronic Pain.” Experimental Neurology 234, no. 2 (2012): 316–329. https://doi.org/10.1016/j.expneurol.2011.09.038.
- Lauren Nicotra, Jonathan Tuke, Peter Grace, Paul Edward Rolan, and Mark Rowland Hutchinson. “Sex Differences in Mechanical Allodynia: How Can It Be Preclinically Quantified and Analysed?” Frontiers in Behavioral Neuroscience 8 (2014): 40. https://doi.org/10.3389/fnbeh.2014.00040.
- Perquin, Christel W, Alice A. J. M Hazebroek-Kampschreur, Joke A. M Hunfeld, Arthur M Bohnen, Lisette W. A van Suijlekom-Smit, Jan Passchier, and Johannes C van der Wouden. “Pain in Children and Adolescents: A Common Experience.” Pain 87, no. 1 (July 1, 2000): 51–58. https://doi.org/10.1016/S0304-3959(00)00269-4.
- Watkins, Linda R., Erin D. Milligan, and Steven F. Maier. “Glial Activation: A Driving Force for Pathological Pain.” Trends in Neurosciences 24, no. 8 (August 2001): 450–55. https://doi.org/10.1016/S0166-2236(00)01854-3.
